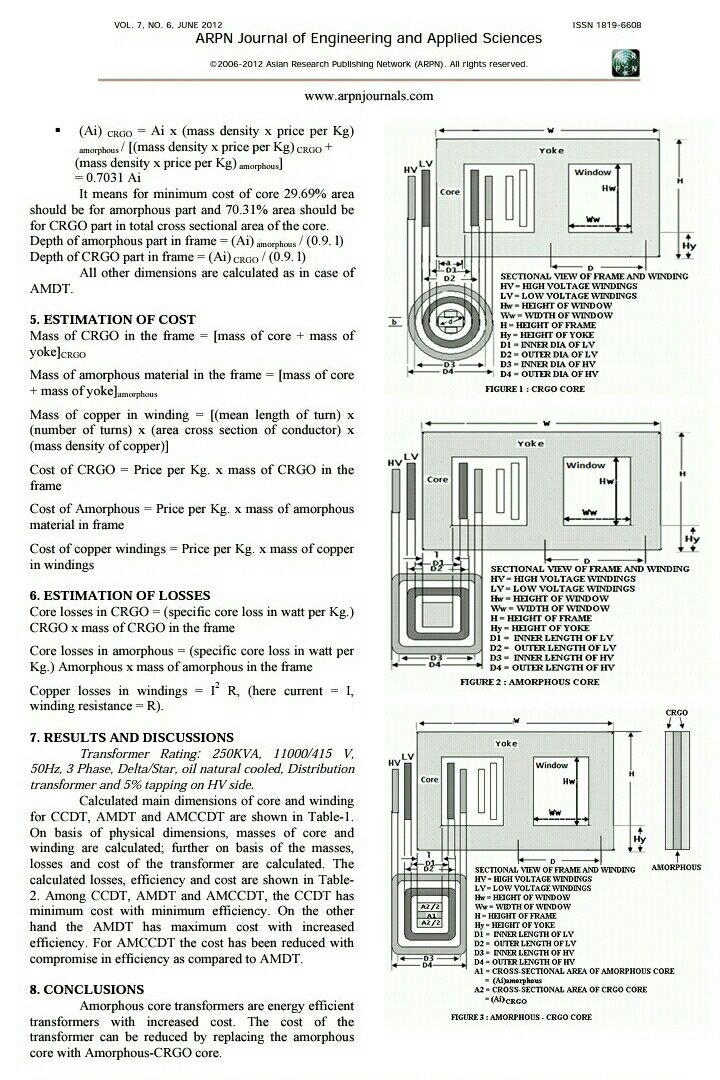
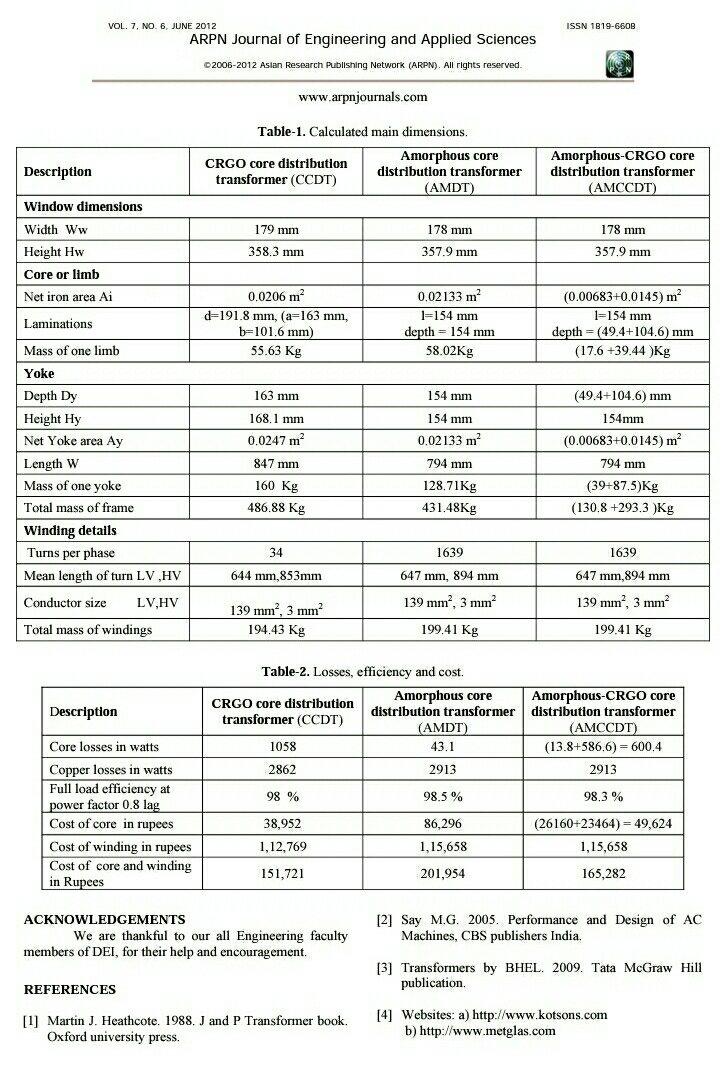
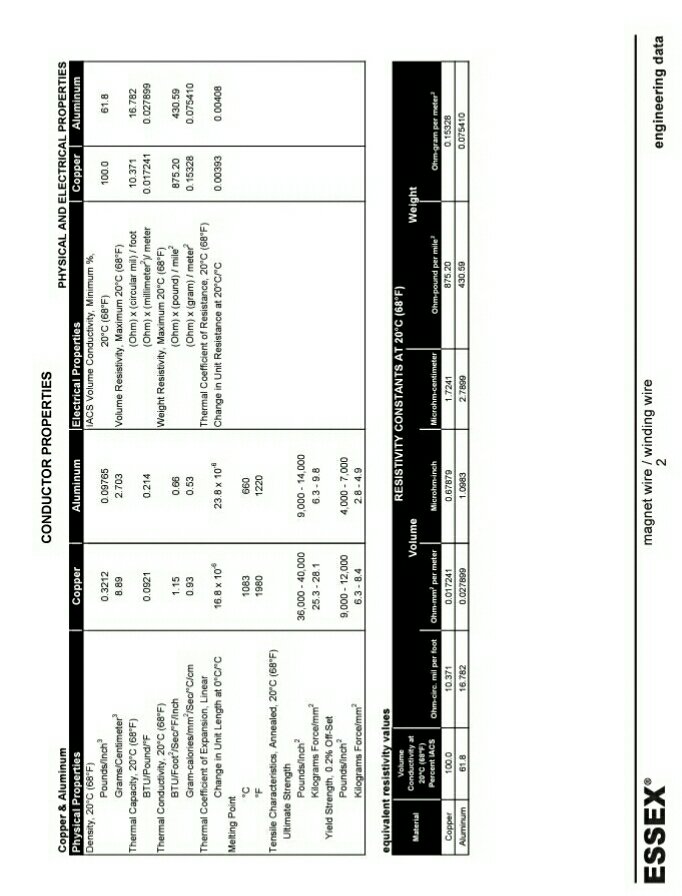
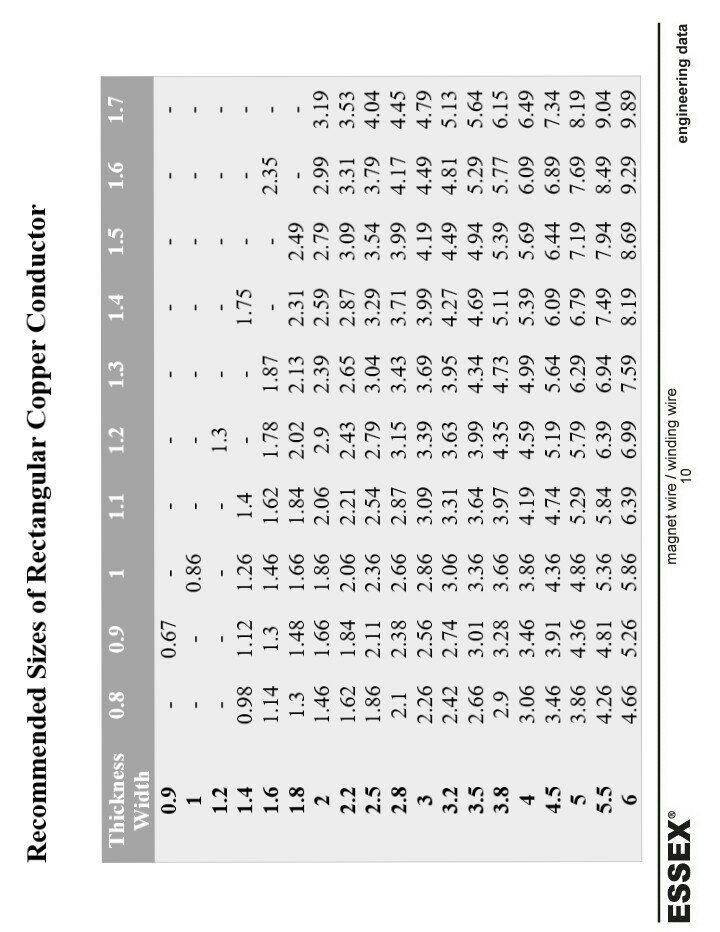
Introduction
Everybody is familiar with the functions that electricity can perform. It can be used for lighting, heating, traction and countless other purposes. The question always arises, “What is electricity” ? Several theories about electricity were developed through experiments and by observation of its behaviour. The only theory that has survived over the years to explain the nature of electricity is the Modern Electron theory of matter. This theory has been the result of research work conducted by scientists like Sir William Crooks, J.J. Thomson, Robert A. Millikan, Sir Earnest Rutherford and Neils Bohr. In this chapter, we shall deal with some basic concepts concerning electricity.
1.1. Nature of Electricity
We know that matter is electrical in nature i.e. it contains particles of electricity viz. protons and electrons. The positive charge on a proton is equal to the negative charge on an electron. Whether a given body exhibits electricity (i.e. charge) or not depends upon the relative number of these particles of electricity.
(i) If the number of protons is equal to the number of electrons in a body, the resultant charge is zero and the body will be electrically neutral. Thus, the paper of this book is electrically neutral (i.e. paper exhibits no charge) because it has the same number of protons and electrons.
(ii) Iffromaneutralbody,some*electronsareremoved,thereoccursadeficitofelectronsin the body. Consequently, the body attains a positive charge.
(iii) If a neutral body is supplied with electrons, there occurs an excess of electrons. Consequently, the body attains a negative charge.
1.2. Unit of Charge
The charge on an electron is so small that it is not convenient to select it as the unit of charge. In practice, coulomb is used as the unit of charge i.e. SI unit of charge is coulomb abbreviated as C. One coulomb of charge is equal to the charge on 625 × 1016 electrons, i.e. 1 coulomb = Charge on 625 × 1016 electrons Thus when we say that a body has a positive charge of one coulomb (i.e. +1 C), it means that the body hasadeficitof625×1016 electrons from normal due share. The charge on one electron is given by ; Charge on electron = – 1.6 × 10–19
1.3. The Electron
Since electrical engineering generally deals with tiny particles called electrons, these small particles require detailed study. We know that an electron is a negatively charged particle having negligible mass. Some of the important properties of an electron are :
(i) Charge on an electron, e = 1.602 × 10–19 coulomb
(ii) Mass of an electron, m = 9.0 × 10–31 kg
(iii) Radius of an electron, r = 1.9 × 10–15 metre
The ratio e/m of an electron is 1.77 × 1011 coulombs/kg. This means that mass of an electron is very small as compared to its charge. It is due to this property of an electron that it is very mobile andisgreatlyinfluencedbyelectricormagneticfields.
1.4. Energy of an Electron
An electron moving around the nucleus possesses two types of energies viz. kinetic energy due to its motion and potential energy due to the charge on the nucleus. The total energy of the electron is the sum of these two energies. The energy of an electron increases as its distance from the nucleus increases.Thus,anelectroninthesecondorbitpossessesmoreenergythantheelectroninthefirst orbit ; electron in the third orbit has higher energy than in the second orbit. It is clear that electrons in the last orbit possess very high energy as compared to the electrons in the inner orbits. These last orbit electrons play an important role in determining the physical, chemical and electrical properties of a material.
1.5. Valence Electrons
The electrons in the outermost orbit of an atom are known as valence electrons. The outermost orbit can have a maximum of 8 electrons i.e. the maximum number of valence electrons can be 8. The valence electrons determine the physical and chemical properties of a material. These electrons determine whether or not the material is chemically active; metal or non-metal or, a gas or solid. These electrons also determine the electrical properties of a material. Onthebasisofelectricalconductivity,materialsaregenerallyclassifiedintoconductors, insulators and semi-conductors. As a rough rule, one can determine the electrical behaviour of a material from the number of valence electrons as under : (i)When the number of valence electrons of an atom is less than 4 (i.e. half of the maximum eight electrons), the material is usually a metal and a conductor. Examples are sodium, magnesium and aluminium which have 1, 2 and 3 valence electrons respectively. (ii)When the number of valence electrons of an atom is more than 4, the material is usually a non-metal and an insulator. Examples are nitrogen, sulphur and neon which have 5, 6 and 8 valence electrons respectively. (iii)When the number of valence electrons of an atom is 4 (i.e. exactly one-half of the maximum 8 electrons), the material has both metal and non-metal properties and is usually a semi-conductor. Examples are carbon, silicon and germanium.
1.6. Free Electrons
We know that electrons move around the nucleus of an atom in different orbits. The electrons in the inner orbits (i.e., orbits close to the nucleus) are tightly bound to the nucleus. As we move away from the nucleus, this binding goes on decreasing so that electrons in the last orbit (called valence electrons) are quite loosely bound to the nucleus. In certain substances, especially metals (e.g. copper, aluminium etc.), the valence electrons are so weakly attached to their nuclei that they can be easily removed or detached. Such electrons are called free electrons. Those valence electrons which are very loosely attached to the nucleus of an atom are called free electrons. The free electrons move at random from one atom to another in the material. Infact, they are so loosely attached that they do not know the atom to which they belong. It may be noted here that all valence electrons in a metal are not free electrons. It has been found that one atom of a metal can provide at the most one free electron. Since a small piece of metal has billions of atoms, one can expect a very large number of free electrons in metals. For instance, one cubic centimetre of copper has about 8.5 × 1022 free electrons at room temperature. (i) A substance which has a large number of free electrons at room temperature is called a conductor of electricity e.g. all metals. If a voltage source (e.g. a cell) is applied across the wire of aconductormaterial,freeelectronsreadilyflowthroughthewire,thusconstitutingelectriccurrent. The best conductors are silver, copper and gold in that order. Since copper is the least expensive out of these materials, it is widely used in electrical and electronic industries. (ii) A substance which has very few free electrons is called an insulator of electricity. If a voltagesourceisappliedacrossthewireofinsulatormaterial,practicallynocurrentflowsthrough the wire. Most substances including plastics, ceramics, rubber, paper and most liquids and gases fall in this category. Of course, there are many practical uses for insulators in the electrical and electronic industries including wire coatings, safety enclosures and power-line insulators. (iii) There is a third class of substances, called semi-conductors. As their name implies, they are neither conductors nor insulators. These substances have crystalline structure and contain very few free electrons at room temperature. Therefore, at room temperature, a semiconductor practically behaves as an insulator. However, if suitable controlled impurity is imparted to a semi-conductor, it is possible to provide controlled conductivity. Most common semi-conductors are silicon, germanium, carbon etc. However, silicon is the principal material and is widely used in the manufacture of electronic devices (e.g. crystal diodes, transistors etc.) and integrated circuits.